
This paper briefly explains the meaning, principle, and mechanism of dispersion and aggregation.
What is dispersion?
What is dispersion?
Dispersion is a process in which pigments and fillers that are insoluble in solvents are crushed (deagglomerated) in a resin solution to a certain particle size using physical forces such as shear forces to obtain uniform and stable particles. The resulting liquid is called a dispersion.In addition to solid(solid phases) and liquid(liquid phases) dispersions, there are also dispersions in which liquids (liquid phases) are dispersed (e.g., water and oil). These are commonly referred to as emulsions, and milk is a type of emulsion in nature.
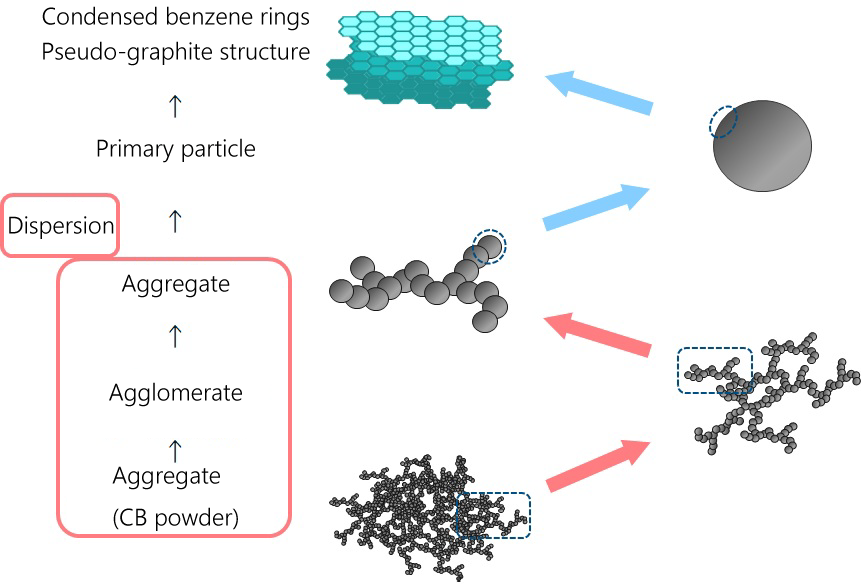
FIG. 1 Dispersion and Agglomeration Model of Carbon Black
Principles and mechanisms of dispersion
Powder particles, such as pigments and fillers, are usually agglomerated from a large number of primary particles.As an initial step in the process of dispersing the agglomerated particles, the surface of the particles is first wetted with a resin solution or the like.
Then the particles are fined using mechanical shear forces. Micronized particles exert a force that tends to agglomerate again, and a stabilized dispersion can be prepared by adding an appropriate additive to suppress this.
What is aggregation?
What is aggregation?
Agglomeration is a large number of primary particles (particles of the smallest unit of pigment or filler) of pigment or filler collect and solidify.Pigments and fillers used as raw materials are usually agglomerated to form dry powders.
In dispersions, whether the particles collect again (re-agglomerate) or stably maintain the dispersed state depends on the intermolecular force (van der Waals force) originally possessed by the pigments, fillers, and other substances, or on the electrical properties of the particle surface during dispersion in the case of aqueous solutions.
It is thought that if the repulsive force, which is offset by the electrical repulsive force on the surface of the particles and the intermolecular attractive force (van der Waals force) between the particles, is greater than the kinetic energy of the particles, the stable dispersion state will continue.
Therefore, the distance between the particles (e.g., the concentration of the dispersion and the particle size) and the pH of the aqueous solution may be factors for stability and reagglomeration.
Stabilisation of the dispersion
In order to stabilize the dispersion, it is necessary to select an appropriate dispersant and add an appropriate amount. The effect of adding a dispersant is steric hindrance.As shown in the figure, polymeric surfactants adsorb to the particles and interfere with agglomeration, thereby stabilizing the dispersion. However, depending on the polarity and pH of the solvent, the dispersant may adversely aid aggregation and cause reagglomeration, and proper formulation is very important.
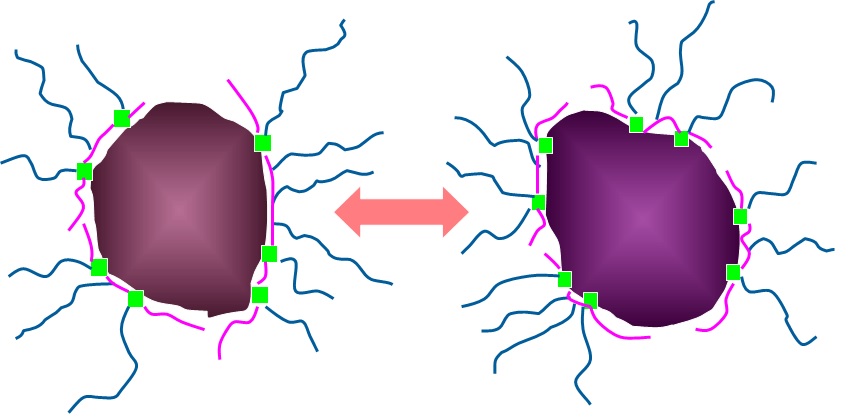
Fig. 2 Steric hindrance model
TOKUSHIKI can perform dispersion processing with optimal formulation depending on the circumstances, based on experiences of dispersion processing over many years.
Fine particle dispersion liquids
Summary
- Dispersion is a process in which pigments and fillers are finely crushed in a resin solution to obtain a uniform and stable state.
- Agglomeration is the clumping of many pigment and filler particles.
- By properly selecting and adding a small amount of a dispersant to stabilize the dispersion, the dispersion is stabilized for a long period of time by the effect of steric hindrance, etc.





